The New England Journal of Medicine (NEJM) today published a peer-reviewed original paper with the results of the landmark clinical trial demonstrating the efficacy of our PRIMA brain computer interface (BCI) retinal implant. The paper showed PRIMA restored functional central vision; 80% of patients demonstrated meaningful improvement of visual acuity and were able to read letters, numbers, and words.
The PRIMA device, comprised of a small light-powered retinal implant and a special pair of glasses that provide wireless power and data to the implant, targets patients suffering from geographic atrophy (GA) due to age-related macular degeneration (AMD), a leading cause of blindness affecting over 5 million people worldwide. It is based on work originally conducted by Professor Daniel Palanker at Stanford University, a co-author of the paper.
“This study confirms that, for the first time, we can restore functional central vision in patients blinded by geographic atrophy,” said Dr. Frank Holz, lead author of the NEJM paper, lead investigator, and Chair of the Department of Ophthalmology at the University Hospital of Bonn. “The implant represents a paradigm shift in treating late-stage AMD.”
“This breakthrough underscores our commitment to pioneering technologies that provide hope to patients in need, and which have the ability to transform lives,” said Max Hodak, founder and CEO of Science. “We are excited about the potential of PRIMA to redefine vision restoration for these patients.”
Watch the story behind the discovery:
Study Highlights
The multi-center clinical trial evaluated the PRIMA implant system in 38 patients across 17 clinical sites in five countries. The key findings include:
- Mean improvement of 25.5 letters (more than 5 lines) on ETDRS letter chart.
- 84% of patients reported the ability to read letters, numbers, and words, restoring functional central vision.
- 80% of patients achieved significant prosthetic visual acuity improvements of at least logMAR 0.2 (equivalent to - 10 letters on ETDRS chart) at 12 months (p<0.001).
- No significant decline in mean existing peripheral natural vision was observed.
- The implant can be safely implanted under the atrophic macula. “Serious adverse events” occurred primarily within the first two months post-implantation and 95% of these resolved within 2 months.
The Data Safety Monitoring Board for the study recommended PRIMA for European market approval, finding that the benefits to patients outweighed the risk of the implantation surgery. In Europe, we have applied for regulatory approval and hope to have PRIMA available to patients next year. In the US, an FDA approval process is underway.
If you or a member of your family has a condition for which PRIMA might be helpful, please consider signing up to be part of our patient registry. If you are a medical professional interested in learning more details about PRIMA’s mechanism of action or the surgical implant technique, please consider joining our professional network.
A Transformational Approach to Vision Restoration
The PRIMA implant is a novel subretinal photovoltaic implant paired with specialized glasses that project near-infrared light to the implant, which acts like a miniature solar panel. A zoom feature provides patients with the ability to magnify letters. The implant rests within the atrophied area of the retina and measures 2mm x 2mm x 30µm. With its ultra-thin profile and seamless, wireless integration, PRIMA offers a solution to those affected by central vision loss.
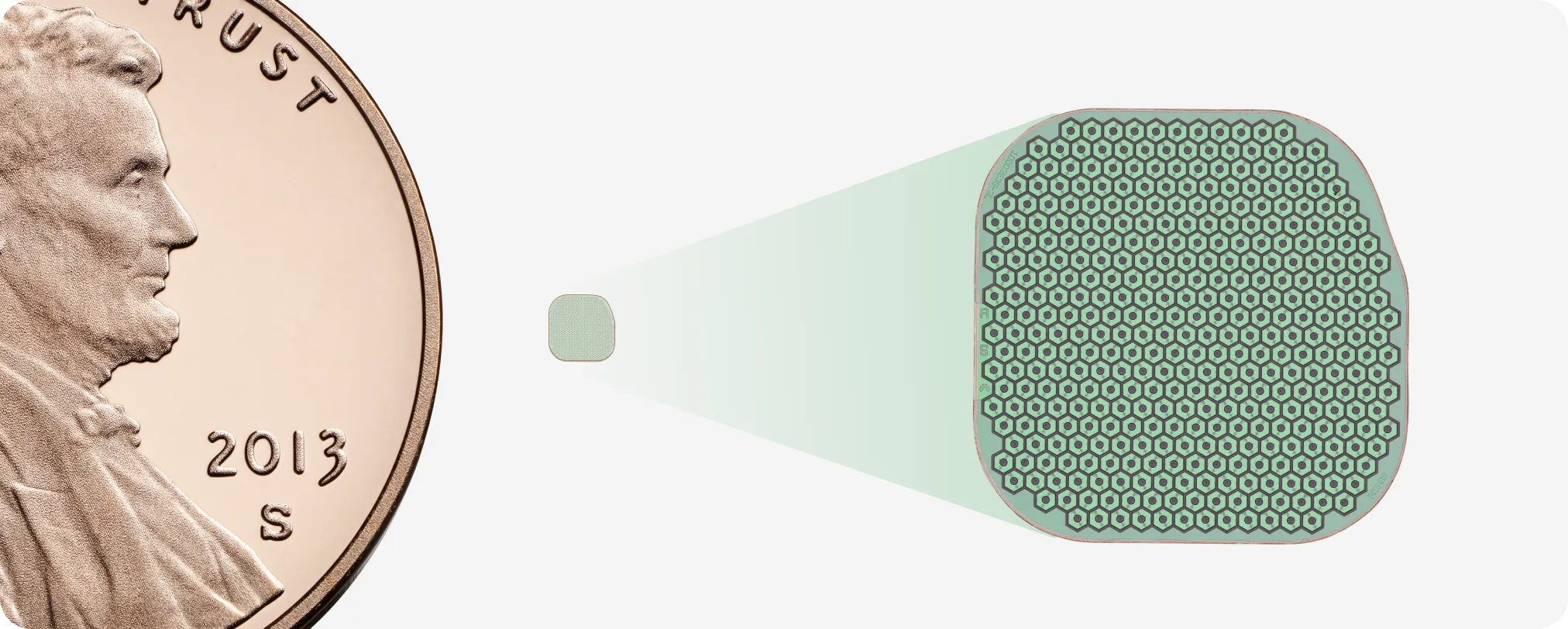
GA is characterized by a loss of photoreceptors in the retina. PRIMA combats this loss with its wireless subretinal implant that operates as an array of artificial photoreceptors, stimulating the remaining cells to carry the visual signal to the brain. Unlike conventional therapies that attempt to slow disease progression, PRIMA directly restores lost functional vision in GA patients.
“It’s the first time that an attempt at vision restoration in these cases has achieved results – and in such a large number of patients. More than 80% of the patients were able to read letters and words, and some of them are reading pages in a book. This is really something we couldn’t have dreamt of when we started on this journey together with Professor Palanker more than a decade ago,” said Prof. José-Alain Sahel, M.D, senior co-author of the paper from the Department of Ophthalmology, University of Pittsburgh School of Medicine, Pittsburgh; Sorbonne Université, Paris; and Institut de la Vision, Paris.
“GA is one of the leading causes of vision loss, and before today, there was nothing which restores that lost vision,” explained Mahi Muqit, PhD FRCOphth, a consultant vitreoretinal surgeon at Moorfields Eye Hospital in London who has performed the procedure multiple times for his patients, and a co-author of the paper. “Patients hear about other types of experimental therapy, such as gene therapy, but they’re all just trying to slow vision loss. Artificial vision is the only approach which actually gives patients any vision back. And when you speak to patients with very severe vision loss, that’s what they want.”
Millions of people have AMD; this generation of PRIMA can treat a subset with advanced GA where vision loss is severe. We are developing the next version of the implant and glasses which will optimize visual performance further with digital image processing and streamlined ergonomics to bring this technology to more patients.
CAUTION: Investigational device. Limited by federal law to investigational use.
