-
Europe
-
Preclinical (R&D)
Completed
-
Feasibility Study
Completed
-
PRIMAvera Study
Completed
-
CE Mark
Ongoing
-
Commercial Launch
-
-
United States
-
Preclinical (R&D)
Completed
-
Feasibility Study
Ongoing
-
Clinical Trial
-
Limited Availability
-
General Availability
-
Macular Degeneration
Age-related macular degeneration (AMD) is a degenerative disease that causes a progressive loss of central vision.
The vision loss is concentrated in the macula, the central area of the retina responsible for high-detail vision for tasks like face recognition or reading. AMD is commonly classified into dry AMD caused by a thinning of the retina as photoreceptors degenerate or wet AMD caused by abnormal blood vessels in the retina.
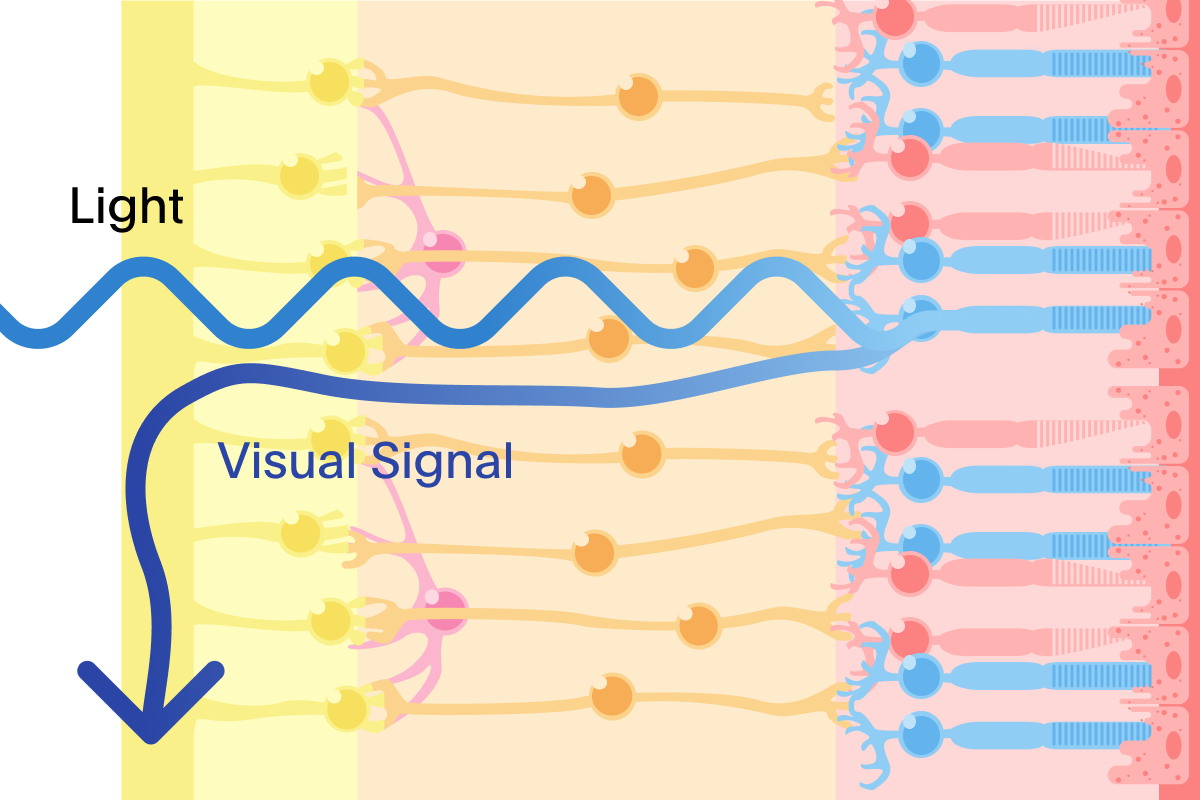
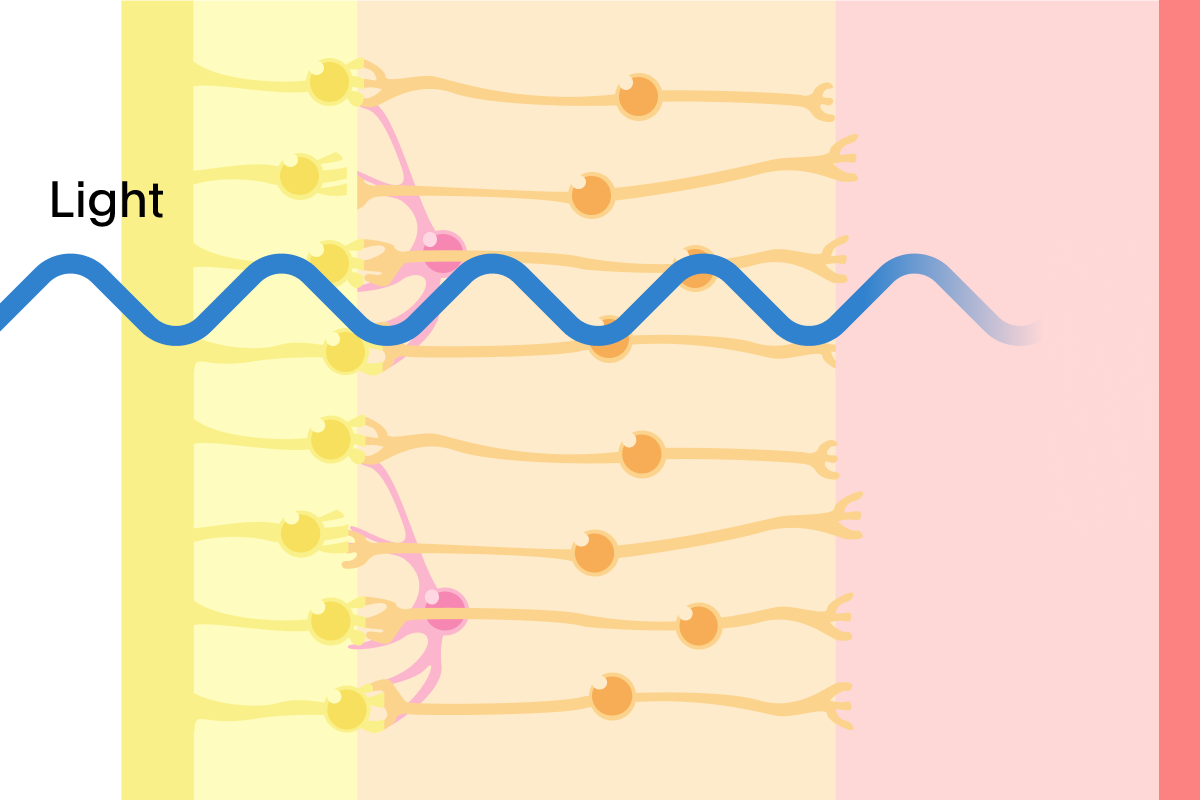

Our Treatment: In clinical trials, PRIMA restored form vision by replacing the function of damaged photoreceptors
Building on decades of pioneering research in miniaturized solar technology, the PRIMA system has two main parts: a small device placed under the retina where the eye's light-sensing cells have been lost; and a pair of glasses that sends both visual data and power in the form of patterned light that is projected on to the implant.
Once implanted, the PRIMA implant uses gentle electrical signals to activate healthy nerve cells in the eye. In our clinical trials, this process enabled some patients to read sequences of letters with a clinically meaningful improvement of letter acuity.
Learn more about PRIMACurrent Status
Science's PRIMAvera clinical study in Europe is indicated for advanced atrophic dry age-related macular degeneration (GA) (NCT04676854). The study is evaluating the safety and efficacy of the PRIMA implant for patients with AMD. Based on the positive preliminary results, we have submitted an application for a CE mark to the European Union. A separate feasibility study in the US continues.
Patient Registry: Science Corporation is actively seeking people interested in learning more about our vision loss treatments.
Join the Patient Registry
More Resources
More information about treatment options, disease details, and current research
