The central problem of brain-computer interfacing is that while you want to measure and manipulate the membrane potential of a neuron, that neuron is far away and embedded in a very complex and fragile biological tissue. The obvious idea of solving this by getting physically close to the neuron is limited by the fact that placing anything into the brain inevitably destroys some amount of brain tissue, even if small (sometimes known as the butcher number).1 Destroying 10,000 cells to record from 1,000 might be perfectly justified if you have a serious injury and those thousand neurons create a lot of value — but it really hurts as a scaling characteristic.
Noninvasive BCIs are concerned with detecting neuronal activity, or a proxy of it, at a distance; ideally through the skull, simply wearable. While there is exciting research ongoing in areas like ultrasound and fMRI, among others, there seem to be real limits to what’s likely achievable relative to an ideal of being able to bidirectionally communicate with hundreds of thousands or millions of perfectly stable single units (neurons). That doesn’t mean that there aren’t important products to be made with noninvasive devices, in particular possibly “therapeutic BCIs” for neuromodulatory-type applications that require comparatively low informational bandwidth, but I don’t think it’s too controversial to say that there’s room at the high end left open.
People forget, but the very first patient to receive a purpose-built brain-computer interface, in 1996, didn’t get a silicon probe or a tungsten microwire – they received a neurotrophic electrode, a little glass cone with chemicals that encouraged neurons to grow in. And it worked! There’s a long history of experiments2 with devices that leverage, in some form, living neurons as part of its function; an idea more recently called a biohybrid neural interface.
At Science, we’re developing a biohybrid probe technology using highly engineered, stem cell-derived neurons embedded in vitro in electronics and engrafted into the brain, forming new biological connections. The basic idea is pretty simple: since the brain is already largely composed of neurons, what happens if we add more neurons? The answer is they grow in and wire up extensively. Importantly, we solve the central problem described above by keeping the cell bodies with us, which is possible because we started with them. Only their axons and dendrites grow out into the brain, joining the representations they encounter.
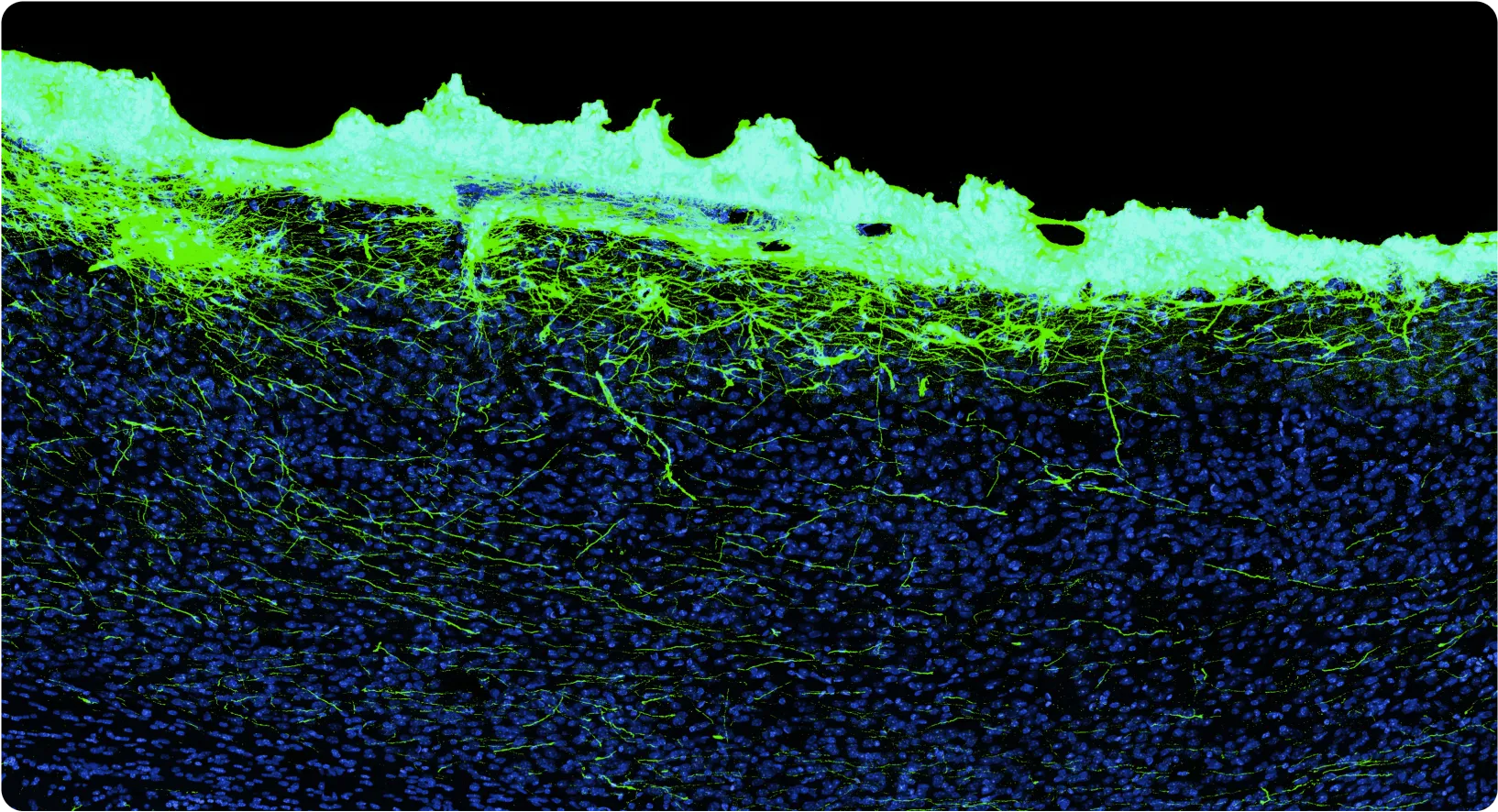
There are a bunch of interesting benefits to this: the signal-to-noise ratio is great, so you can use smaller amplifiers and burn less power. Also, critically, you get chemical synapses, so you can imagine using neurons of various types — say some dopaminergic neurons, some acetylcholinergic neurons, some glutamatergic neurons — and then being able to “stimulate” using specific neurotransmitters. Since neurons form many connections, engrafting a million neurons (an easily practical device volume, far less than a cubic millimeter) might produce over a billion synapses; this is very far from thinking about things in terms of “channels” of electrodes. There is an immense amount of science to do here.
Today we’re releasing our first paper on our biohybrid work, Optogenetic Stimulation of a Cortical Biohybrid Implant Guides Goal Directed Behavior, by Jennifer Brown et al., from the Science Team, available on bioRxiv. It covers an early feasibility study we did to convince ourselves this could work, and represents only a small part of what we’ve done. We think that this approach is scalable up to millions of neurons and eventually capable of bandwidth on par with the fiber bundle that connects the two hemispheres of the brain.
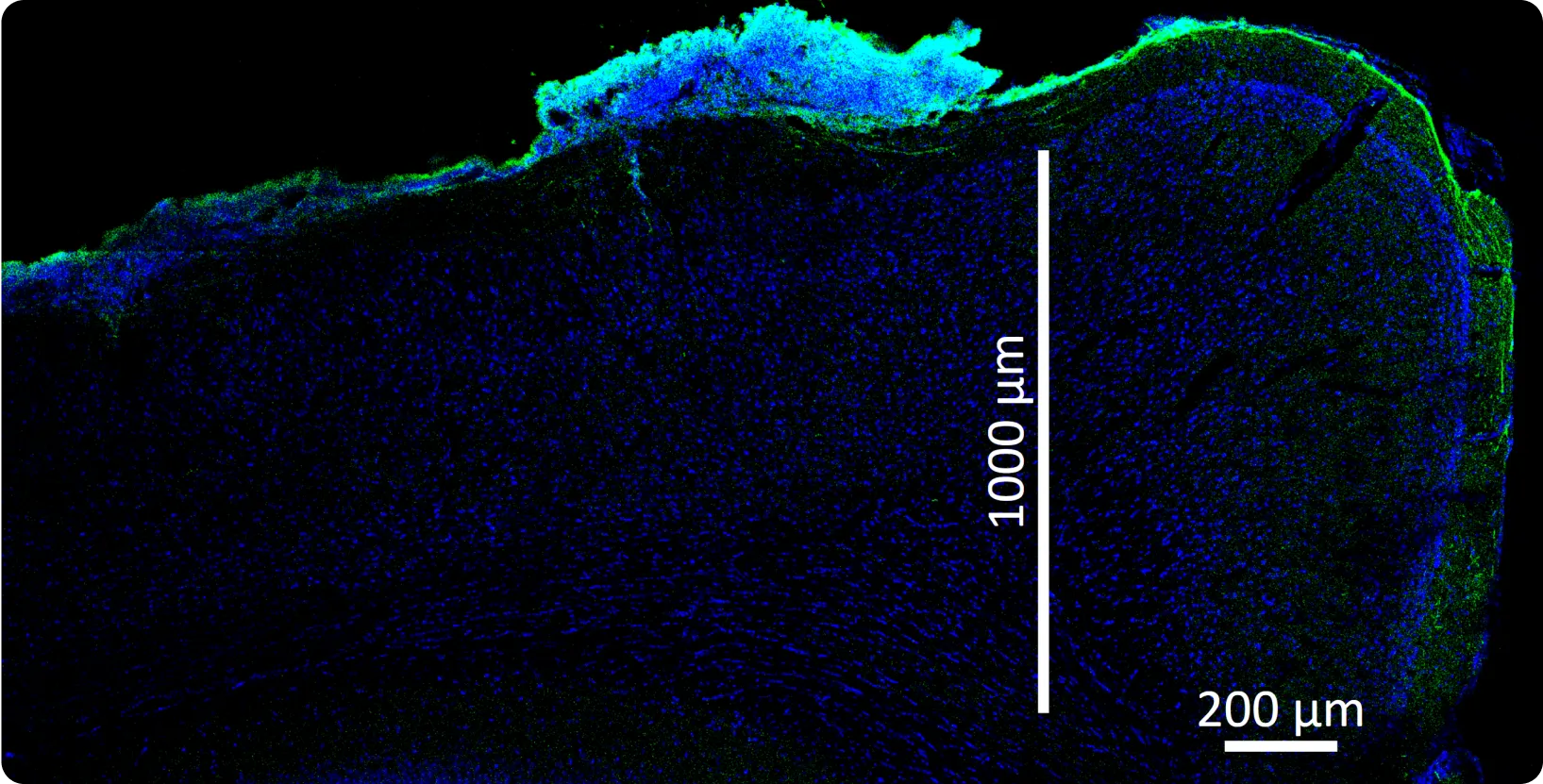
There were a bunch of papers we took as clues that this could work. In 2015, Blake Byers at Stanford was working on developing an animal model of Parkinson’s, and took a skin sample from a patient which he reprogrammed into neurons and engrafted into a rat. When the graft cells were excited, he found clear activation at distant locations in the brain, showing that they had functionally integrated into the circuit. In 2019, Amy Rochford at Cambridge (now at Science) wrote a review of biohybrid neural interfaces and identified four distinct types of the idea. Sunil Gandi’s work on functional neuronal graft transplantation and D. Kacy Cullen’s work on graft axon “living electrodes” are also notable.
More broadly, there’s a sizable literature out there from physicians who treated a patient or two with neural tissue transplants in attempts to treat various diseases over the years. They consistently report that the grafts often appear to survive and integrate, and, interestingly, nothing noticeable typically changes with the patient.
While the idea has been around, there are very steep technical challenges to developing a real production or clinical grade biohybrid system. For example, it’s important that the graft cells are compatible with the patient’s immune system so that it doesn’t reject them. It is possible to make a biohybrid graft from a patient’s own cells (“autologously”) but it would take many months and cost over a million dollars per patient. What you really want is a “hypoimmunogenic allogeneic” line which is a fancy expression for “cloaked from the immune system and compatible with anyone of the same species.” Making hypoimmunogenic stem cells is not trivial and a major undertaking in its own right, with multiple companies focused on it specifically. Engendering graft survival inside the device and post-implantation is a major project; neurons are very fragile cells, and are being asked to survive glycemic shock, hypoxia, and the host immune system, all while undergoing maturation inside an active electronic device with (hopefully) just the right type of glial support.
What we end up with is a pretty futuristic artifact: a physical cell, descended in an unbroken line of divisions back to an initial donor fibroblast two years ago, that we’ve been editing ever since. There is no easy way to just print more; these cells have been selected through a long chain of low-likelihood events, making them an unusual kind of extremely atoms product in our increasingly bits world.
Biohybrid technology has an important and exciting role to play in the future of brain-computer interfaces and neural engineering broadly. Relative to other more mature approaches, it has a low technology readiness level, but the challenges appear surmountable and the promise is enormous.
Our biohybrid work is being led by Alan Mardinly and Yifan Kong, two of our cofounders. Alan’s team produces the cells; Yifan’s team produces the devices, with support from the Integrated Circuits group led by Selim Eminoglu and the Science Foundry led by Kara Zappitelli.
Our primary focus as a company remains bringing our PRIMA retinal prosthesis to patients. With the success of the PRIMAvera clinical trial, we are eager to submit our CE mark application in Europe; we’re hard at work preparing over a thousand pages of regulatory documentation for that. We’re also actively exploring transitioning our US feasibility study into a pivotal trial to bring the PRIMA technology to the US. Our research efforts on biohybrid BCIs, and other areas, support our long term mission as we continue to look for new ways to understand and communicate with the brain.
Read more about our biohybrid neural interface technology in development.
1. There are limits to how small an electrode can be made and still be effective. They also typically degrade over time, though progress has been made there.
2. We've included some references to earlier work by others in this vein on our biohybrid technology page.
