Science has agreed to acquire assets from Pixium Vision SA, including three ongoing clinical trials in late-stage macular degeneration
I had the opportunity to see something incredible a few months ago: a video of a blind patient reading. There have been many attempts at restoring vision by electrically stimulating the retina, but those devices could only cause fleeting flashes of light called phosphenes. With more electrodes you might get more phosphenes, but I (and many others) have always had the concern that a field of phosphenes is not really vision.
Indeed: earlier patients might be able to reason their way through piecing lines into letters, but they weren’t able to intuitively string them together into words, much less words into sentences. Pixium’s PRIMA implant is, to my knowledge, the first really definitive demonstration of what researchers call “form vision” (the ability to recognize visual elements as parts of a larger object) in humans.
For a patient who has lost the light-sensitive cells in the eye – the photoreceptors – but for whom the retina is otherwise intact such as in many rod-cone dystrophies, there are two principal cell types one might consider targeting to restore the flow of visual information into the brain.
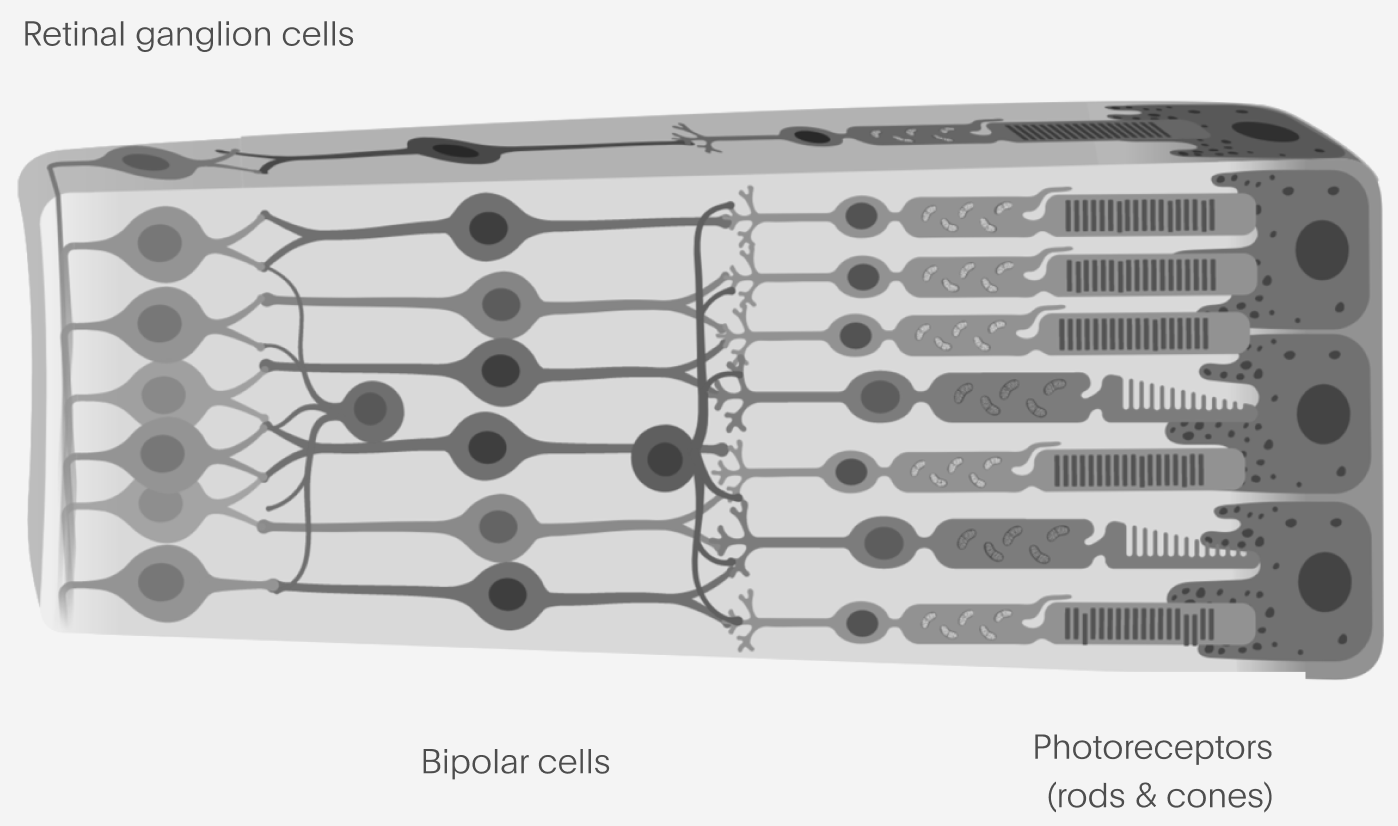
Retinal ganglion cells, or RGCs, represent the tightest informational bottleneck of vision into the brain: the outputs of the RGCs form the “optic nerve” which connects the eye into the rest of the brain. These are the output layer of the retina, where we would be theoretically least sensitive to whatever disease state is present in the eye. RGCs are also readily targetable by existing AAV-based gene therapy technologies. On the other hand, the information representation found in RGCs (how they encode visual information) is complex and there are extremely stringent requirements for any device that might be able to excite them selectively enough to reproduce such an encoding.
At the other end, bipolar cells are the first synapse past the photoreceptors. They have the advantage of having much simpler representations compared to the RGCs, something much closer to the bitmapped image excited in the photoreceptors. But, they aren’t a true neuron (they use graded potentials, not spikes, most of the time), which makes optogenetics more complex. Accessing them with a device usually means placing something under the retina, which is a potentially riskier procedure compared to placing something only in the vitreous chamber or on the retinal surface.
There were reasons to believe that bulk electrical stimulation of dozens or hundreds of bipolar cells wouldn’t produce form vision. There are many different “types” of bipolar cells which convey different pieces of information, and in any case they may be too degraded in late-stage disease that warranted an implant or gene therapy. Thanks to Pixium’s preliminary study results, we know this is not the case, and activating the bipolars – even largely non-selectively – can at least in some patients restore meaningful vision. The retina is in many ways an ideal special case for this kind of electrical stimulation, and there’s a clear pathway to further performance improvements in future versions of the device.
The Pixium device works by being, in effect, a tiny solar panel with hundreds of tiny little sub-panels. If you hit one of these pixels with infrared light, you will excite a small electric field directly above the point you illuminated. Wearing glasses with a camera and a small laser projector, an image can be projected onto the implant, exciting the remaining bipolar cells, and thereby getting information back into the signal chain that ultimately leads to vision in the brain.
Following acceptance of our bid by the French commercial court and French Foreign Direct Investment approval, Science has agreed to acquire the assets of Pixium Vision SA. Pixium has three ongoing clinical trials with the PRIMA device, including a 38-patient pivotal study in Europe (NCT04676854). This transaction also significantly strengthens our IP portfolio. Our goal is to continue and expand these studies to bring this device to patients.
I want to recognize the extraordinary efforts of the Pixium team to get the PRIMA device to where it is now; it is literally several peoples’ life’s work. We were highly impressed by their team, including Ralf, Karine, Brian, Alexandra, Offer and Lloyd, and we are very thankful for the work they did to help us understand the Pixium technology and ultimately make a deal possible.
PRIMA came out of a concept originally developed by Prof. Daniel Palanker at Stanford, and we’re excited to collaborate with him on the next generations of this device family.
What does this mean for our optogenetic gene therapy and the Science Lightsheet? It’s too early to know which approach, electrical or optogenetic, will work best in the retina long term as these technologies mature. But whichever is used 20 years from now, there is a huge amount of good all of these early devices can do today. They represent very different approaches that make different tradeoffs and at least initially will probably target different patient subpopulations. We would love to be able to offer a range of products to match each individual patient’s needs with the best of what’s available over time.
Science now has best-in-class therapies targeting both the RGCs and bipolars. Through these and other new technologies we pursue, we are committed to bringing meaningful restoration of useful vision to late stage rod-cone dystrophy patients globally as soon as possible.
